The Federal Trade Commission is taking action against California-based Precision Patient Outcomes, Inc. and the company’s CEO Margrett Priest Lewis for marketing an over-the-counter dietary supplement containing nothing more than vitamins, zinc, and a flavonoid as an effective treatment to mitigate the effects of COVID-19.
“The FTC will halt baseless claims about COVID-19 treatments that harm consumers’ pocketbooks and health,” said Samuel Levine, Director of the Commission’s Bureau of Consumer Protection. “We don’t simply seek to stop this kind of fraud, but to permanently prohibit companies and company owners engaging in misconduct from endangering the health and well-being of American consumers.”
In its complaint, the FTC is seeking to permanently stop the company and its CEO from using deceptive treatment or prevention claims with no supporting scientific evidence to sell their dietary supplement. The FTC alleges that these practices violate the FTC Act and the COVID-19 Consumer Protection Act. The latter allows the Commission to seek civil penalties in cases of COVID-related consumer fraud.
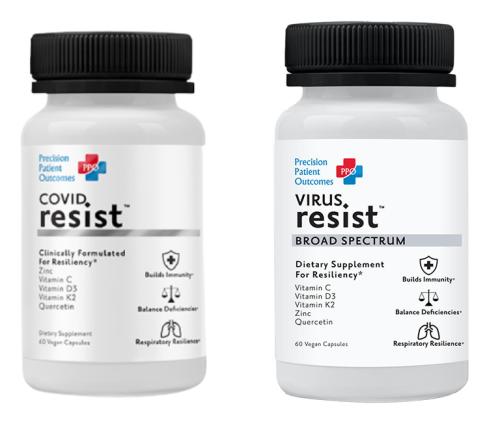
Precision Patient Outcomes, based in Berkeley, California, developed, labeled, advertised, marketed, distributed, and sold a supplement under the names COVID Resist and VIRUS Resist, to consumers nationwide during the COVID pandemic. According to the FTC’s complaint, in May 2021 the defendants began advertising and marketing COVID Resist on the company’s website and social media pages with deceptive claims that the product can treat, prevent, or mitigate COVID-19.
Lewis, the company’s co-founder and CEO, formulated the product and is in direct control of Precision Patient Outcomes’ business operations. Among other things, she has been actively involved in promoting COVID Resist and VIRUS Resist using the company’s website; through posts on Facebook, Instagram, and TikTok; and on her personal social media accounts.
Despite knowing about the Commission’s enforcement action under the COVID-19 Consumer Protection Act against a company making similar claims about the science and efficacy of its products, the defendants only changed the name of the product from COVID Resist to VIRUS Resist and continued to deceptively advertise it as an effective treatment for COVID-19.
The Commission vote authorizing the staff to file the complaint was 4-1, with Commissioner Christine S. Wilson dissenting. The complaint was filed in the U.S. District Court for the Northern District of California.
The Federal Trade Commission works to promote competition and protect and educate consumers. The FTC will never demand money, make threats, tell you to transfer money, or promise you a prize. Learn more about consumer topics at consumer.ftc.gov, or report fraud, scams, and bad business practices at ReportFraud.ftc.gov. Follow the FTC on social media, read consumer alerts and the business blog, and sign up to get the latest FTC news and alerts.
